 Compliance and Legal Matters
Compliance and Legal Matters
International projects may be subject to both domestic and foreign laws, which can quickly complicate a project if caught unaware. Issues often triggering legal matters include: establishing legal status abroad, tax filing and auditing requirements, hiring foreign workers, physical space rentals, technology transfer and export issues, animal and human subject research, sub-contracting, international partnership agreements, intellectual property, and commercialization. Some of these issues will be discussed below, while others will be discussed in the Doing Business section.
 Legal Registration
Legal Registration
If your institution has many projects operating within a particular foreign country or if a project anticipates hiring foreign personnel, signing contracts, or establishing a long-term presence in the country, an official “legal presence” may need to be established.
 International Regulations
International Regulations
When assisting principal researchers in international endeavors, international regulations play an important role in determining how the work is completed and how it is reported to the participating institutions and applicable funding agencies. Learn about if and which overarching regulations may apply to global collaborations in this section.
 Export Control
Export Control
Export Controls are U.S. laws that regulate, for reasons of foreign policy and national security, the distribution of strategically important technology, services and information to foreign nationals and foreign countries. Export controls most often become a concern for universities and research institutions when researchers and staff work on projects that are considered controlled or have publication restrictions that include foreign persons or foreign collaborators. Discover how research administrators serve as a resource to identify potential issues of export control at the earliest possible stage of a research project.
 Intellectual Property and Commercialization
Intellectual Property and Commercialization
Intellectual property and commercialization are two issues that are especially salient at the international level – especially when industry partners are involved. They are a central issue in international research collaborations and should be addressed by a legal professional at the earliest chance in a burgeoning project.
 Managing Risk – Contracts and Partnerships
Managing Risk – Contracts and Partnerships
International partnerships, as they relate to research administrators, can be between universities, industrial enterprises, research institutions, national governments, Foundations, and any myriad of other entities. Navigating national and international regulations, cultural complexities, infrastructural challenges, and language barriers are just some of the things to consider when signing international contracts and partnerships. Find helpful resources here.
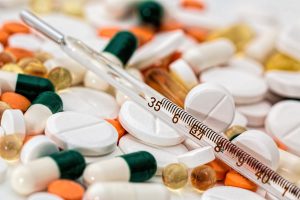 Clinical Trials, Human, and Animal Subjects
Clinical Trials, Human, and Animal Subjects
Clinical trials and the inclusion of human and animal subjects in international research projects can bring a hefty amount of additional reporting requirements, red tape, and cultural misunderstandings. This page broaches legal challenges that accompany global research and offers insight into issues that should be addressed early on in the project.
 Templates, Presentations, and Other Resources
Templates, Presentations, and Other Resources
Are you wondering what not to do on a project? Have a story where everything went as expected (does this Holy Grail exist within global collaborations)? Did you learn something important in an instance that you would like to share with the community? Find these cases and other “I wish I knew…” examples here.
